Animal Cell Wiki Biography
Source:- Google.com.pk
The term Molecular biology was first used by Warren Weaver in 1938. Molecular biology is the study of molecular underpinnings of the processes of replication, transcription, translation, and cellular function.
A typical animal cell. Within the cytoplasm, the major organelles and cellular structures include: (1) nucleolus (2) nucleus (3) ribosome (4) vesicle (5) rough endoplasmic reticulum (6) Golgi apparatus (7) cytoskeleton (8) smooth endoplasmic reticulum (9) mitochondria (10) vacuole (11) cytosol (12) lysosome (13) centriole.
Contents [hide]
1 Macromolecules
1.1 Carbohydrates
1.1.1 a.Monosaccharides
1.1.2 b.Disaccharide
1.1.3 c.Oligosaccharide
1.1.4 d.Polysaccharides
1.2 Proteins
1.3 Lipids
1.4 Water
1.5 Noncovalent bond
2 pH plays important role in living organism
3 Cell The basic unit of life
3.1 Prokaryotes
3.2 Eukaryotic cell
3.3 Plant cell is different from animal cell
3.4 Origin of Eukaryotic organelles and endosymbiotic theory
3.4.1 Eukaryotic organelles
3.4.2 Prokaryotic organelles
4 Macromolecules which are present in the cell membrane
5 Facts to be remembered
6 Question time
7 References
Macromolecules[edit]
The term macromolecule was coined by Nobel laureate Hermann Staudinger in the 1920s, although his first relevant publication on this field only mentions high molecular compounds (in excess of 1,000 atoms). At that time the phrase polymer as introduced by Berzelius in 1833 had a different meaning from that of today: it simply was another form of isomerism for example with benzene and acetylene and had little to do with size.Some examples of organic macromolecules are bio-polymers (carbohydrates, proteins and lipids, Polysaccharides) and synthetic polymers (plastics, synthetic fiber and rubber).
Carbohydrates[edit]
A carbohydrate (kɑ:bəˈhaɪdreɪt/) is an organic compound which has the empirical formula Cm(H2O)n; that is, consists only of carbon, hydrogen and oxygen, with a hydrogen:oxygen atom ratio of 2:1 (as in water). Carbohydrates can be viewed as hydrates of carbon, hence their name. Structurally however, it is more accurate to view them as polyhydroxy aldehydes and ketones.Historically nutritionists have classified carbohydrates as either simple or complex, however, the exact delineation of these categories is ambiguous. Today, simple carbohydrate typically refers to monosaccharides and disaccharides and complex carbohydrate meeans polysaccharides (and oligosaccharides).
a.Monosaccharides[edit]
Monosaccharides (from Greek monos: single, sacchar: sugar) are the most basic units of biologically important carbohydrates. They are the simplest form of sugar and are usually colorless, water-soluble, crystalline solids. Some monosaccharides have a sweet taste. Examples of monosaccharides include glucose (dextrose), fructose (levulose), galactose, xylose and ribose. Monosaccharides are the building blocks of disaccharides such as sucrose and polysaccharides (such as cellulose and starch). Further, each carbon atom that supports a hydroxyl group (except for the first and last) is chiral, giving rise to a number of isomeric forms all with the same chemical formula. For instance, galactose and glucose are both aldohexoses, but have different chemical and physical properties.
b.Disaccharide[edit]
A disaccharide or biose is the carbohydrate formed when two monosaccharides undergo a condensation reaction which involves the elimination of a small molecule, such as water, from the functional groups only. Like monosaccharides, disaccharides also dissolve in water, taste sweet and are called sugars.The glycosidic bond can be formed between any hydroxyl group on the component monosaccharide. So, even if both component sugars are the same (e.g., glucose), different bond combinations (regiochemistry) and stereochemistry (alpha- or beta-) result in disaccharides that are diastereoisomers with different chemical and physical properties. Depending on the monosaccharide constituents, disaccharides are sometimes crystalline, sometimes water-soluble, and sometimes sweet-tasting and sticky-feeling.
Disaccharide Unit 1 Unit 2 Bond
Sucrose (table sugar, cane sugar, beet sugar, or saccharose) glucose fructose α(1→2)
Lactulose galactose fructose β(1→4)
Lactose (milk sugar) galactose glucose β(1→4)
Maltose glucose glucose α(1→4)
Trehalose glucose glucose α(1→1)α
Cellobiose glucose glucose β(1→4)
c.Oligosaccharide[edit]
An oligosaccharide (from the Greek oligos, a few, and sacchar, sugar) is a saccharide polymer containing a small number (typically three to ten of component sugars, also known as simple sugars (monosaccharides). Oligosaccharides can have many functions; for example, they are commonly found on the plasma membrane of animal cells where they can play a role in cell-cell recognition.In general, they are found either O- or N-linked to compatible amino acid side-chains in proteins or to lipid moieties . e.g.Fructo-oligosaccharides (FOS), which are found in many vegetables, consist of short chains of fructose molecules. (Inulin has a much higher degree of polymerization than FOS and is a polysaccharide.) Galactooligosaccharides (GOS), which also occur naturally, consist of short chains of galactose molecules. These compounds can be only partially digested by humans.
d.Polysaccharides[edit]
Glycogen.
Polysaccharides are polymeric carbohydrate structures, formed of repeating units (either mono- or di-saccharides) joined together by glycosidic bonds. These structures are often linear, but may contain various degrees of branching. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure, these macromolecules can have distinct properties from their monosaccharide building blocks. They may be amorphous or even insoluble in water.Starches are glucose polymers in which glucopyranose units are bonded by alpha-linkages. It is made up of a mixture of Amylose (15–20%) and Amylopectin (80–85%). Amylose consists of a linear chain of several hundred glucose molecules and Amylopectin is a branched molecule made of several thousand glucose units (every chain 24–30 glucose unit). Starches are insoluble in water. They can be digested by hydrolysis, catalyzed by enzymes called amylases, which can break the alpha-linkages (glycosidic bonds). Humans and other animals have amylases, so they can digest starches. Potato, rice, wheat, and maize are major sources of starch in the human diet. The formation of starches are the way that plants store glucose.Glycogen is a polysaccharide that is found in animals and is composed of a branched chain of glucose residues. It is stored in liver and muscles.Chitin is one of many naturally occurring polymers. It is one of the most abundant natural materials in the world. Over time it is bio-degradable in the natural environment. Its breakdown may be catalyzed by enzymes called chitinases, secreted by microorganisms such as bacteria and fungi, and produced by some plants.Arabinoxylans are the copolymers of two pentose sugars - arabinose and xylose.
Proteins[edit]
Proteins are polymer of amino acids linked together by peptide bonds. Amino acids can be divided into two group essential amino acids and non-essential amino acids. Proteins and carbohydrates contain 4 kcal/gram as opposed to lipids which contain 9 kcal/ gram. The liver, and to a much lesser extent the kidneys, can convert amino acids used by cells in protein biosynthesis into glucose by a process known as gluconeogenesis. The essential amino acids, which must be obtained from external sources (food), are leucine, isoleucine, valine, lysine, threonine, tryptophan, methionine, phenylalanine and histidine. On the other hand, non-essential amino acids are synthesized in our body from other amino acids. The non-essential amino acids are arginine, alanine, asparagine, aspartic acid, cysteine, glutamine, glutamic acid, glycine, proline, serine, and tyrosine. Proteins( /ˈproʊtiːnz/; also known as polypeptides) are organic compounds made of amino acids arranged in a linear chain and folded into a globular or fibrous form. The amino acids in a polymer are joined together by the peptide bonds between the carboxyl and amino groups of adjacent amino acid residues. The sequence of amino acids in a protein is defined by the sequence of a gene, which is encoded in the genetic code.Proteins were first described by the Dutch chemist Gerhardus Johannes Mulder and named by the Swedish chemist Jöns Jakob Berzelius in 1838
Animal Cell Wiki Animal Cell Model Diagram Project Parts Structure Labeled Coloring and Plant Cell Organelles Cake


Animal Cell Wiki Animal Cell Model Diagram Project Parts Structure Labeled Coloring and Plant Cell Organelles Cake
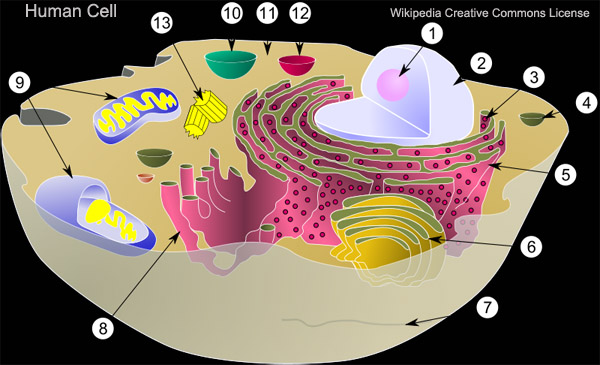

Animal Cell Wiki Animal Cell Model Diagram Project Parts Structure Labeled Coloring and Plant Cell Organelles Cake


Animal Cell Wiki Animal Cell Model Diagram Project Parts Structure Labeled Coloring and Plant Cell Organelles Cake


Animal Cell Wiki Animal Cell Model Diagram Project Parts Structure Labeled Coloring and Plant Cell Organelles Cake
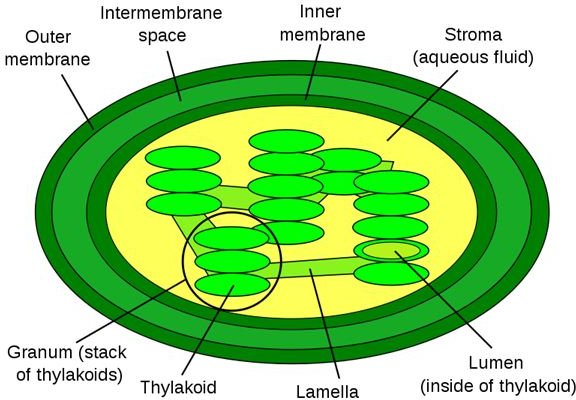

Animal Cell Wiki Animal Cell Model Diagram Project Parts Structure Labeled Coloring and Plant Cell Organelles Cake


Animal Cell Wiki Animal Cell Model Diagram Project Parts Structure Labeled Coloring and Plant Cell Organelles Cake
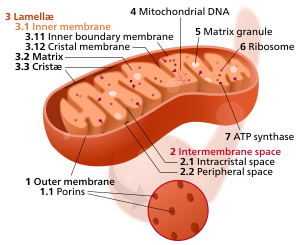

Animal Cell Wiki Animal Cell Model Diagram Project Parts Structure Labeled Coloring and Plant Cell Organelles Cake
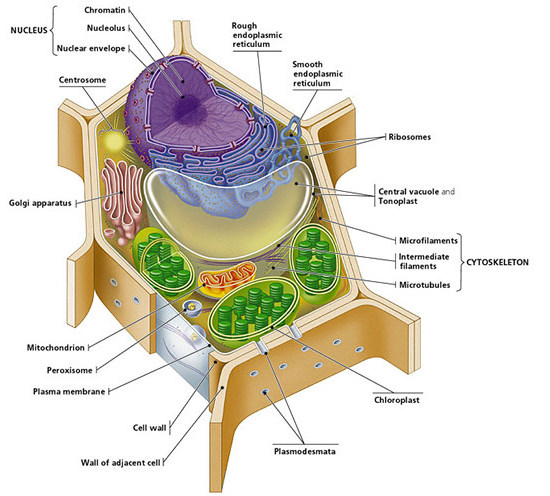

Animal Cell Wiki Animal Cell Model Diagram Project Parts Structure Labeled Coloring and Plant Cell Organelles Cake


Animal Cell Wiki Animal Cell Model Diagram Project Parts Structure Labeled Coloring and Plant Cell Organelles Cake


Animal Cell Wiki Animal Cell Model Diagram Project Parts Structure Labeled Coloring and Plant Cell Organelles Cake
No comments:
Post a Comment